BIS certification for Aluminium and aluminium alloy foil for pharmaceutical packaging IS 16011:2012
BIS certification is mandatory for aluminium and aluminium alloy foils for pharmaceutical packaging. As Per IS 16011:2012 The versatility of wrought aluminium and aluminium alloy bars, rods, tubes, sections, plates and sheets, due to their ease of fabrication and recycling, increases their utility in various engineering fields.
In the field of electrical engineering, aluminium and its alloys play an important role in various applications such as electrical enclosures, busbars, generators, transformers, motors and power transmission and distribution systems. Their indispensable properties including electrical conductivity, lightweight nature and corrosion resistance make important contributions to the design and construction of power systems, as well as promote sustainability by reducing energy consumption and environmental impact.
The stringent requirements outlined in IS 16011:2012 for wrought aluminium and aluminium alloy bars, rods, tubes, sections, plates and sheets used in electrical applications include mechanical properties, electrical resistivity/conductivity and chemical composition. This standard applies to extruded angles, channels, various sections, profiles, tubes and hollow sections with a thickness of 3 mm or more, as well as extruded bars and rods with a diameter of more than 6 mm.
Key highlights
| Product Name | Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging — Specification |
| Applicable Indian Standard | IS 16011: 2012 |
| Applicable Certification Scheme | Product Certification Scheme (ISI Mark Scheme) Scheme 1 - Schedule 2 |
| Compliance Requirement | Mandatory |
| QCO Link | Quality Control Order |
| Scope as per Standard | This standard covers the requirements of aluminium and aluminium alloy-bare / coated / laminated foil for pharmaceutical packaging applications. It is applicable for 0.020 mm (20 µm) to 0.040 mm (40 µm) foil thicknesses. |
Tests
The following are the major tests for Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging:
- Pin Hole Count
- Freedom from Defects
- Average Thickness of bare foil
- Coating/Lamination
- Width
- Bursting Strength
- Peel Strength
- Sealing Strength
- Material
- Lubricants
A notable aspect is the mandatory role of the ISI Mark, which serves as identification of compliance with the exact standards of IS 16011:2012. Without this certification, marketing, import or export of these products in the Indian consumer market is prohibited.
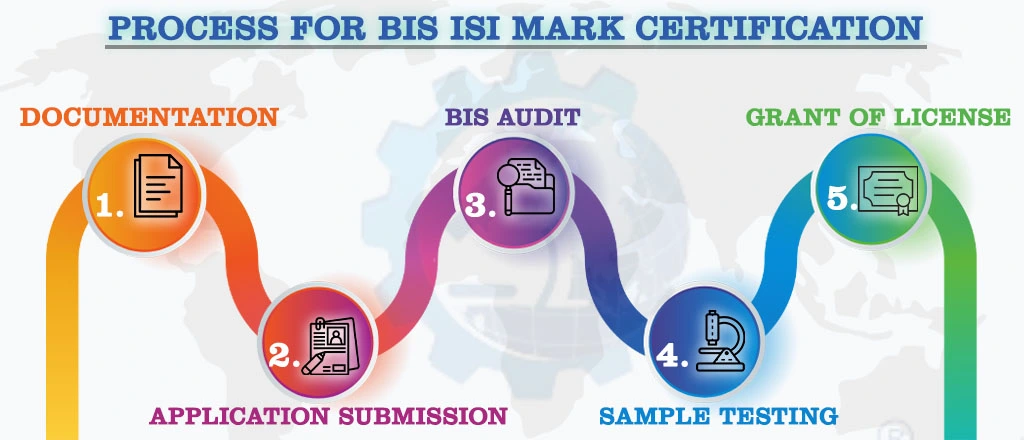
BIS Certification Process
Obtaining a BIS license involves a thorough assessment of manufacturing infrastructure, quality control capabilities, testing facilities, and production processes. This comprehensive evaluation ensures that the products not only comply with regulations but are also safe and reliable for consumers.
NOTE:
For Detailed Information about the Procedure for BIS ISI Certification, Visit :
Timeline for BIS Certification
The approximate timeline to obtain BIS Certification for Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging. to use ISI mark as Per IS 16011:2012 is given below:
- For Indian Manufacturers (Standard Timeframe – 30 days)
- For Foreign Manufacturers (Standard Timeframe – 180 days)
Conclusion:
The implementation of the Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging — Specification (Quality Control) Order, 2023, is ushering in a transformative era for the market of Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging — Specification in India. Manufacturers, by embracing these guidelines and securing BIS certification, are not merely complying with regulations; they are setting new benchmarks for excellence in the industry. Opting for BIS-certified Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging — Specification signifies more than a product purchase; it represents a commitment to dependability, superior quality, and safety.
BIS certification for Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging — Specification goes beyond meeting regulatory checkboxes, assuring the highest performance standards in these products. In an era where quality takes precedence, choosing BIS-certified Aluminium and Aluminium Alloy Foil for Pharmaceutical Packaging — Specification guarantees a product that is not only dependable but also of the finest quality.
In this landscape of evolving standards, Aleph INDIA stands as a leading BIS consultant in India, dedicated to assisting manufacturers and importers in obtaining BIS licenses for their products. With a commitment to excellence, Aleph INDIA ensures that your products not only meet but exceed the required quality benchmarks. Aleph INDIA has been serving the industry as a single-window operator for all product regulatory compliance. We can assist importers or manufacturers in meeting all criteria for importing or selling a product in the Indian market.
International Audits & Participation
Testimonials
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
View All Services
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.