BIS CERTIFICATION FOR CLINICAL THERMOMETERS IS 3055 (Part1)
Published Date: January 22, 2025 4 min Read
BIS certification is essential for Clinical Thermometers, Part 1: Solid Stem Type as per IS 3055 (Part 1). This standard ensures that clinical thermometers meet precise accuracy, durability, and safety requirements, which are critical for reliable temperature measurement in healthcare settings. Solid stem clinical thermometers are commonly used in medical environments to accurately monitor body temperature, an essential parameter for diagnosing and treating various health conditions. Compliance with IS 3055 (Part 1) reflects a manufacturer’s commitment to producing high-quality, safe, and dependable clinical thermometers.
IS 3055 (Part 1): Clinical Thermometers, Part 1: Solid Stem Type
The IS 3055 (Part 1) standard provides detailed guidelines on the design, performance, and testing of solid stem clinical thermometers. It ensures that these thermometers deliver precise temperature readings, with stringent specifications for accuracy, range, and response time. The standard outlines requirements for materials, construction, and calibration methods to ensure reliability and prevent malfunction under normal usage conditions. Additionally, it defines the necessary performance tests to verify that the thermometers maintain accuracy over time and across various environmental conditions.
Benefits of BIS Certification for Clinical Thermometers
Mandatory BIS certification under IS 3055 (Part 1) guarantees that solid stem clinical thermometers meet the highest standards of accuracy, safety, and durability. For healthcare professionals, it provides assurance that the thermometers they use will provide reliable readings for patient care. For manufacturers, BIS certification enhances market credibility, ensures compliance with Indian regulations, and builds customer trust by demonstrating a commitment to quality and precision in medical instruments.
Key highlights
| Product Name | Clinical thermometers, Part 1: solid stem type |
| Applicable Indian Standard | IS 3055 (Part1) |
| Applicable Certification Scheme | Product Certification Scheme (ISI Mark Scheme) Scheme 1 - Schedule 2 |
| Compliance Requirement | Mandatory |
| Quality Control Order | Click here | Scope as per Standard | This standard specifies the materials, construction, performance, and testing requirements for low-pressure single or two-stage regulators used with liquefied petroleum gas (LPG) mixtures in the vapor phase. These regulators are designed to operate with an outlet pressure of up to 4.903 kN/m² (50 gf/cm² or 500 mm water column, WC). |
Note
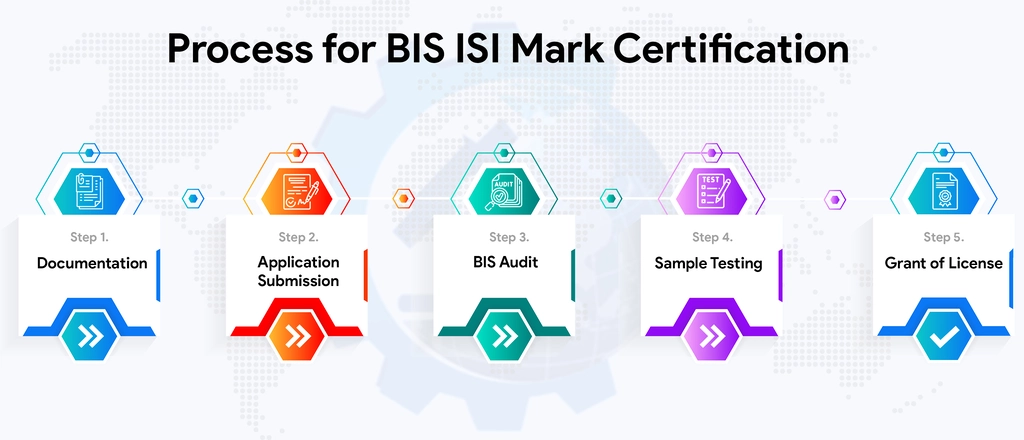
For Detailed Information about the Procedure for BIS ISI Certification, Visit :
Timeline for BIS Certification
The estimated timeline for obtaining BIS certification under IS 3055 (Part 1) is as follows:
- For Indian Manufacturers (Standard Timeframe – 30 days)
- For Foreign Manufacturers (Standard Timeframe – 180 days)
Conclusion
BIS certification under IS 3055 (Part 1) is crucial for ensuring the accuracy, safety, and reliability of solid stem clinical thermometers used in medical environments. Manufacturers adhering to this standard provide healthcare providers with instruments that meet rigorous performance criteria, which are vital for effective patient care.
Partner with Aleph INDIA for expert guidance in obtaining BIS certification and ensuring compliance with national standards for medical temperature measurement devices.
Frequently Asked Questions
International Audits & Participation
Testimonials
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
View All Services
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.