BIS CERTIFICATION FOR FOOD FOR SPECIAL MEDICAL PURPOSE INTENDED FOR INFANTS IS 17945
Published Date: January 04, 2025 4 min Read
Introduction
BIS certification is mandatory for Food for Special Medical Purpose Intended for Infants, governed by the standards outlined in IS 17945:2022. This standard ensures that these specialized products meet critical nutritional requirements and are safe for consumption by infants with specific medical needs. Such food products play an essential role in providing tailored nutrition to support the growth and well-being of infants with medical conditions. By adhering to IS 17945:2022, manufacturers confirm their commitment to the highest levels of quality, safety, and nutritional adequacy.
IS 17945:2022 – Food for Special Medical Purpose Intended for Infants
The standard IS 17945:2022 establishes comprehensive guidelines for the formulation, production, and packaging of food products designed for infants with medical conditions. It outlines specific nutritional compositions, including precise levels of proteins, fats, vitamins, and minerals tailored to meet the unique requirements of these infants. Quality control measures are emphasized to prevent contamination and ensure safety during production. Additionally, the standard mandates the use of tamper-proof, food-grade packaging to maintain the product’s quality and integrity throughout storage and transportation. Compliance with IS 17945:2022 guarantees the reliability and premium quality of these specialized infant food products.
Benefits of BIS Certification for Food for Special Medical Purpose Intended for Infants
Mandatory BIS certification under IS 17945:2022 verifies that these products comply with stringent safety and quality standards. Certified products meet all regulatory requirements, reflecting the manufacturer’s dedication to delivering reliable nutrition for infants with medical conditions. This certification provides assurance to parents and healthcare providers regarding the safety and efficacy of the product. For manufacturers, it enhances brand credibility and ensures compliance with national regulations.
Key highlights
| Product Name | Food for Special Medical Purpose Intended for Infants |
| Applicable Indian Standard | IS 17945:2022 |
| Applicable Certification Scheme | Product Certification Scheme (ISI Mark Scheme) Scheme 1 - Schedule 2 |
| Compliance Requirement | Mandatory |
| Quality Control Order | Click here | Scope as per Standard | This standard prescribes the types, requirements, methods of test and sampling for Food for Special Medical Purpose Intended for Infants. |
Note
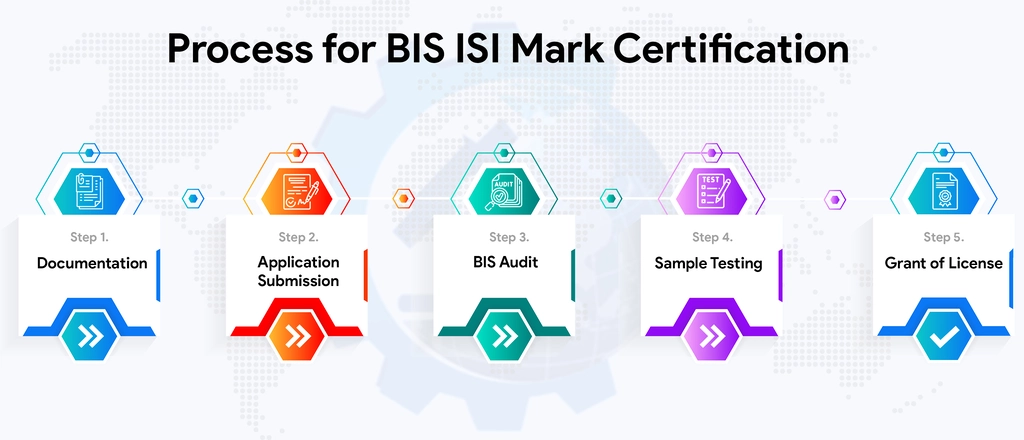
For Detailed Information about the Procedure for BIS ISI Certification, Visit :
Timeline for BIS Certification
The approximate timeline to obtain BIS Certification for IS 17945:2022 is as follows:
- For Indian Manufacturers (Standard Timeframe – 30 days)
- For Foreign Manufacturers (Standard Timeframe – 180 days)
Conclusion
BIS certification under IS 17945:2022 is a critical benchmark for ensuring the safety, nutritional adequacy, and quality of food products intended for infants with special medical needs. For manufacturers, achieving this certification highlights their commitment to regulatory compliance and the provision of premium quality products for infant health. Adhering to this standard reflects a focus on supporting the unique dietary requirements of vulnerable infants, meeting the expectations of health-conscious parents and caregivers.
For expert guidance on obtaining BIS certification, connect with Aleph INDIA and ensure your products comply with the highest standards of excellence and regulatory requirements.
Frequently Asked Questions
International Audits & Participation
Testimonials
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
View All Services
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.