BIS CERTIFICATION FOR MEDICAL TEXTILES NONWOVEN WIPES
IS 17787:2021
In this competitive scenario, it isn't easy to survive in the market without a standard quality and certified product. BIS license may also be required to sell products in the Indian market.
To get BIS certification and produce a standard quality product, the manufacturer must ensure that their product must follow the specified Indian standard.
Let's take a closer look at IS 17787:2021 for medical textiles – nonwoven wipes.
Medical textiles – nonwoven wipes are covered under IS 17787:2021. This standard cover the minimum performance requirements for single-use nonwoven dry or wet wipes for baby care and personal hygiene (baby, adult, facial, skin etc.). This standard does not apply to wet wipes impregnated with alcohol or wipes with a germicidal claim (numerical germ kill, disinfection etc.).
This standard does not address the need for chemicals, cleaning agents, moisturizing agents, aroma, preservatives, and other additives to be impregnated/coated on the product. The various types of chemicals/ingredients used in these items must be within the acceptable limits set by the FDA (Food and Drugs Authority of India) or another regularity authority for the purpose.
The non-woven fabric used in the manufacture of wipes must comply with IS 17788: 2021. The wipes must be clean and free of any compounds that could cause tendering during storage. The length and width of the wipes must be agreed upon by both the buyer and seller. The wipes must be manufactured in hygienic circumstances. Annex B of the standard contains general guidance for good manufacturing practice in order to maintain hygiene requirements at manufacturing facilities. Wipes must meet the specifications mentioned in the standard.
tests
The following test shall be carried out.
- Length and width
- pH
- Total viable count
- Pseudomonas aeruginosa
- Staphylococcus aureus
- Candida albicans
- E. coli
The wipes must be packed securely so that they may be handled and transported normally without tearing and exposing the contents. Each packet of wipes must be legibly and indelibly labelled with the information as specified in the standard.
Conforming to the requirements of this standard, the product(s) may be certified under the terms of the Bureau of Indian Standards Act, 2016 and the Rules and Regulations formulated thereunder, and the product(s) may be marked with the Standard Mark (ISI Mark). The Manufacturer must obtain a BIS license from the Bureau of Indian Standards to use a standard mark (ISI Mark). The BIS grants a license based on a successful assessment of manufacturing infrastructure, quality control, testing capabilities, and production process.
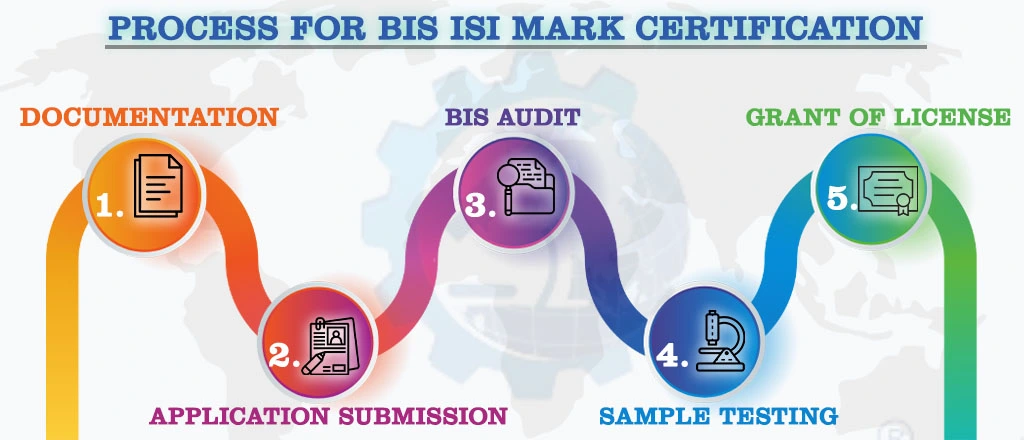
NOTE:
For Detailed Information about the Procedure for BIS ISI Certification
Visit :
• ISI Mark Certification for Domestic Manufacturers• ISI Mark Certification for Foreign Manufacturers
Conclusion:
If a product falls under the scope of the BIS Conformity Assessment Scheme, All the manufacturers, importers, and foreign entities must obtain BIS ISI Certification. The Bureau may cancel the License if the product fails to meet certification requirements.
Aleph INDIA has been serving the industry as a single-window operator for all product regulatory compliance. We can assist importers or manufacturers in meeting all criteria for importing or selling a product in the Indian market.
International Audits & Participation
Testimonials
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
View All Services
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.