BIS CERTIFICATION FOR SINGLE VOLUME PIPETTES
IS 1117:2018
In this competitive scenario, it isn't easy to survive in the market without a standard quality and certified product. BIS licenses may also be required to use standard ISI Mark and sell products in the Indian market.
To obtain BIS certification and produce a standard quality product, the manufacturer must ensure that their product must comply with the specified Indian standard.
Let's take a closer look at IS 1117:2018 for Single Volume pipettes.
IS 1117:2018 covers Laboratory glassware - Single Volume pipettes. The Bureau of Indian Standards adopted this Indian Standard on the recommendation of the Glass, Glassware, and Laboratory Ware Sectional Committee and with the approval of the Chemical Division Council.
This standard specifies the sampling and testing requirements and methods for one-mark pipettes intended for general laboratory use. Single-volume pipettes must be made of glass with chemical resistance and thermal properties at least equal to HGB3 in accordance with ISO 719, be as free of visible defects as possible, and be reasonably free of internal stress. A BIS license for Laboratory glassware Single Volume pipettes is granted to use the Standard Mark in accordance with IS 1117:2018/ ISO 648: 2008.
One-mark pipettes shall be classified into two accuracy classes, A and B. Nominal capacities and accuracy must meet standards. The pipettes must be made of colourless glass with no discernible tint. They must be as free of visible defects as possible and reasonably free of internal strain. The pipe must meet the standard's dimensional requirements and the workmanship and finish requirements. It is required to maintain a laboratory that is adequately staffed and resourced to conduct the various tests outlined in the standard.
TESTS
Tests shall be carried out in accordance with the method specified.
- Maximum permissible errors
- Material
- Dimensions
- Delivery time
- Graduation line
Packing and Marking:
Each one-mark pipette must be marked in accordance with IS 1117:2018. A standard Mark can be applied to each pipette. The pipettes must be packed as agreed upon by the buyer and supplier. All production that meets the Indian Standard and is licensed should bear the standard ISIMark. To use a standard mark, the manufacturer must obtain a BIS license for one mark pipette from the Bureau of Indian Standards. The Bureau grants a BIS license based on a successful assessment of the manufacturing infrastructure, quality control and testing capabilities, and production process. A BIS certification for one-mark pipettes is required to ensure that the product meets the requirements of applicable Indian standards.
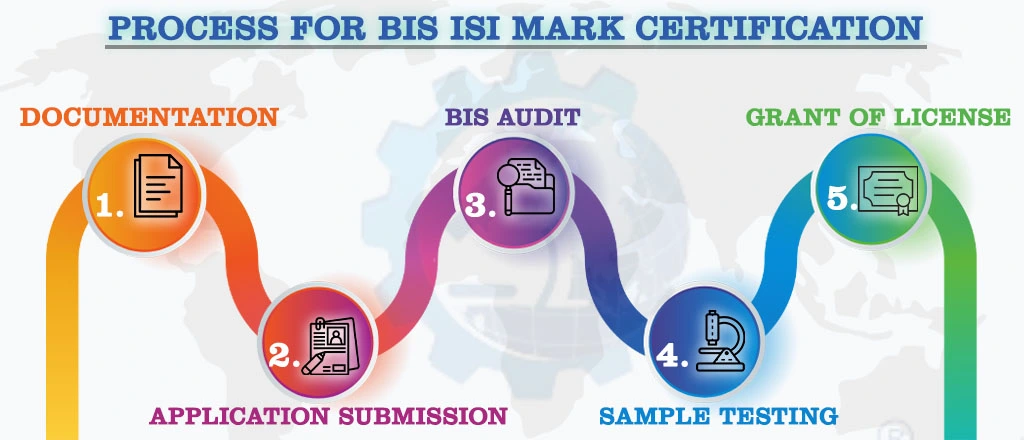
NOTE:
For Detailed Information about the Procedure for BIS ISI Certification, Visit :
Conclusion:
If a product falls under the scope of the BIS Conformity Assessment Scheme, All the manufacturers, importers, and foreign entities must obtain BIS ISI Certification. The Bureau may cancel the License if the product fails to meet certification requirements.
Aleph INDIA has been serving the industry as a single-window operator for all product regulatory compliance. We can assist importers or manufacturers in meeting all criteria for importing or selling a product in the Indian market.
Successful Audits
International Audits & Participation
Testimonials
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
View All Services
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.