BIS CERTIFICATION FOR SAFETY REQUIREMENTS FOR DIAGNOSTIC MEDICAL X-RAY EQUIPMENT: PART 1 IS 7620 (Part 1)
Published Date: January 22, 2025 4 min Read
BIS certification is essential for diagnostic medical X-ray equipment as per IS 7620 (Part 1): 1986. This standard establishes comprehensive safety requirements to ensure the safe design, construction, and performance of diagnostic X-ray equipment, which is critical for minimizing radiation risks to patients and healthcare professionals. Compliance with IS 7620 (Part 1): 1986 demonstrates a manufacturer's commitment to safety and reliability in medical imaging technology.
IS 7620 (Part 1): Safety Requirements for Diagnostic Medical X-ray Equipment
The IS 7620 (Part 1): 1986 standard specifies safety guidelines for diagnostic X-ray equipment used in medical imaging. It outlines requirements for radiation protection, electrical safety, mechanical stability, and operational reliability. The standard ensures that the equipment is designed to reduce radiation exposure, provide accurate diagnostic results, and operate safely in clinical environments. It also specifies performance evaluation methods to verify compliance with safety norms.
Benefits of BIS Certification for Diagnostic Medical X-ray Equipment
Mandatory BIS certification under IS 7620 (Part 1): 1986 ensures that X-ray equipment adheres to the highest safety standards, reducing potential risks associated with radiation exposure and electrical hazards. For healthcare providers, this certification guarantees safer diagnostic practices, enhancing patient trust and care quality. For manufacturers, BIS certification confirms adherence to Indian regulatory standards, improves market credibility, and ensures product acceptance across healthcare facilities. Certified equipment symbolizes a commitment to safety, precision, and compliance with national standards.
Key highlights
| Product Name | Safety requirements for diagnostic medical X-ray equipment: Part 1 |
| Applicable Indian Standard | IS 7620 (Part 1) : 1986 |
| Applicable Certification Scheme | Product Certification Scheme (ISI Mark Scheme) Scheme 1 - Schedule 2 |
| Compliance Requirement | Mandatory |
| Quality Control Order | Click here | Scope as per Standard | This standard (Part 1) specifies the general and safety requirements for all types of diagnostic medical X-ray equipment and their components. It covers equipment with a capacity of up to 500 mA and 150 kV, designed to operate directly on a single-phase power supply or through a phase converter. |
Note
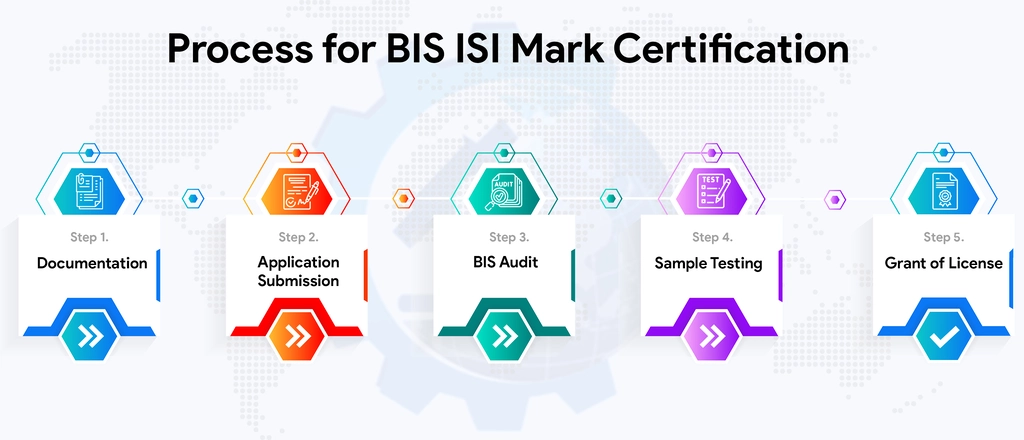
For Detailed Information about the Procedure for BIS ISI Certification, Visit :
Timeline for BIS Certification
The estimated timeline for obtaining BIS certification under IS 7620 (Part 1): 1986 is as follows:
- For Indian Manufacturers (Standard Timeframe – 30 days)
- For Foreign Manufacturers (Standard Timeframe – 180 days)
Conclusion
BIS certification under IS 7620 (Part 1): 1986 is crucial for ensuring the safety and reliability of diagnostic medical X-ray equipment. By complying with this standard, manufacturers contribute to safer medical imaging practices and uphold stringent quality norms in healthcare technology.
Partner with Aleph INDIA for expert assistance in obtaining BIS certification, ensuring compliance with safety standards, and delivering reliable and secure diagnostic solutions.
Frequently Asked Questions
International Audits & Participation
Testimonials
BIS REGISTRATION FOR ELECTRONIC & IT PRODUCT
In the era of globalization, world trade is growing rapidly and henceforth, Manufacturing and Import/Export businesses are also growing drastically...View More
BIS CERTIFICATE FOR FOREIGN MANUFACTURER
The Economy of India-the fastest developing economy on the globe with the capabilities that help it matches up with the biggest international...View More
PRODUCT CERTIFICATION SCHEME (ISI MARK) FOR DOMESTIC MANUFACTURERS
Anything a person buys from food to cars, clothes to electronics, branded to unnamed products there is always a question that wanders in one’s...View More
WIRELESS PLANNING AND COORDINATION (WPC)
WPC: Wireless means communication done from one point to another point without the wires and cables. Electromagnetic waves carry the ...View More
BUREAU OF ENERGY EFFICIENCY (BEE) CERTIFICATE
BEE CERTIFICATE: Energy is the future, and its conservation is the way of the bright future. Everyone claims the environment is important...View More
E-WASTE MANAGEMENT
E-waste is one of the world's fastest-growing trash streams. We currently manufacture almost 50 million tones of it each year...View More
View All Services
Request a call back.
Would you like to speak to one of our Senior Technical advisers over the phone? Just submit your details and we’ll be in touch shortly. You can also email us if you would prefer.