The Central Drugs Standard Control Organization (CDSCO) serves as India’s national regulatory authority for pharmaceuticals, medical devices, and cosmetics. Functioning under the Ministry of Health & Family Welfare, CDSCO is responsible for enforcing the provisions of the Drugs and Cosmetics Act to ensure that only safe, effective, and high-quality healthcare products reach the Indian market.
As the Central Drug Authority, CDSCO operates through an extensive network of six zonal offices, four sub-zonal offices, 13 port offices, and seven state-of-the-art laboratories. Its headquarters oversees regulatory functions such as granting approvals for new drugs, authorizing clinical trials, managing the import of drugs, coordinating with expert committees like the Drugs Consultative Committee (DCC) and Drugs Technical Advisory Board (DTAB), and issuing key licenses as the Central License Approving Authority.
At its core, CDSCO acts as the nation’s healthcare gatekeeper safeguarding and promoting public health by ensuring that every drug, cosmetic, and medical device manufactured, imported, or distributed in India adheres to strict standards of safety, efficacy, and quality. Mandatory compliance with CDSCO regulations not only protects consumers but also strengthens trust and integrity across India’s healthcare ecosystem.
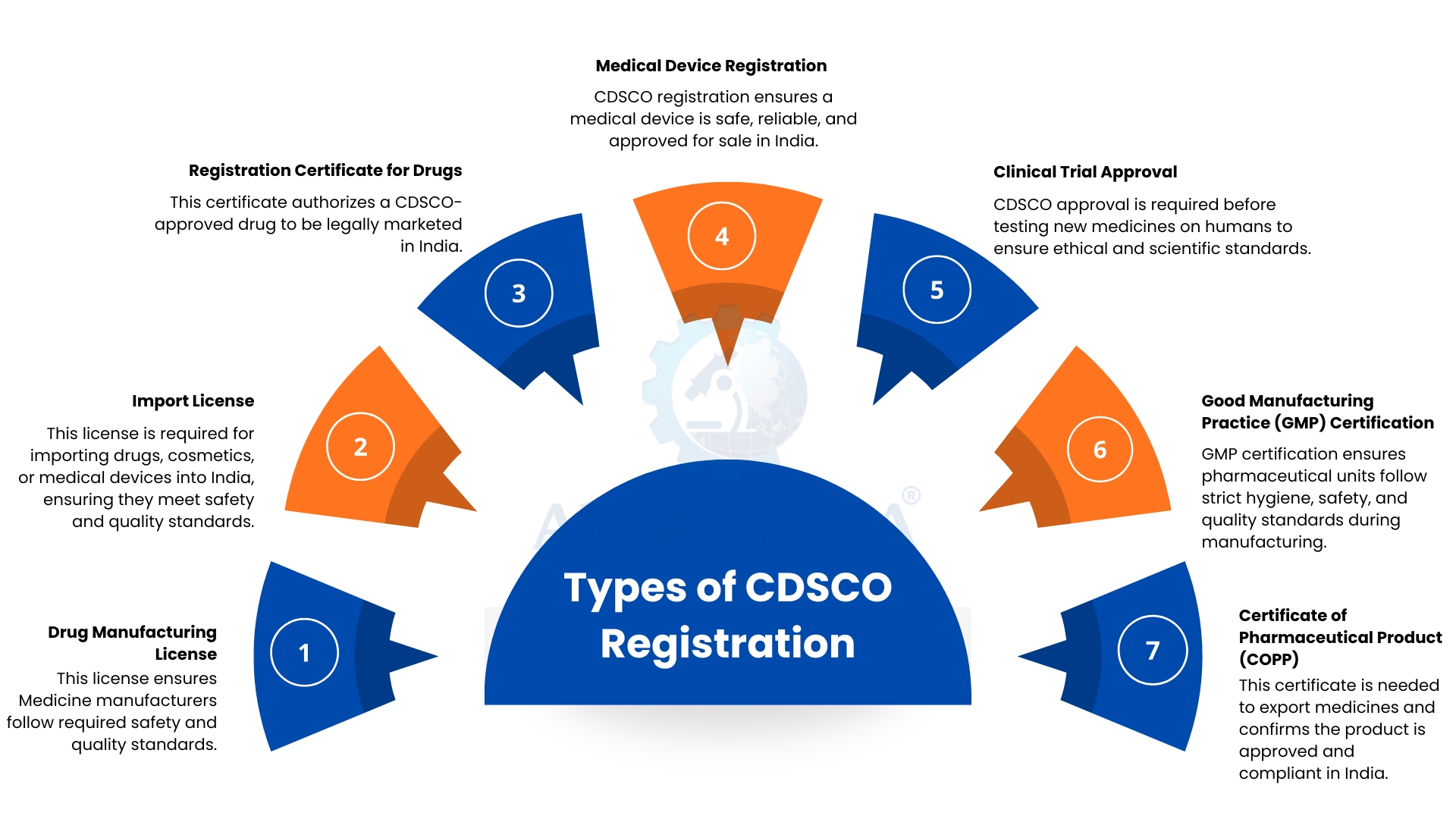
Types of CDSCO Registration
CDSCO offers different types of registrations depending on the product category. These registrations help manufacturers and importers meet compliance requirements before their products enter the Indian market.
1. Drug Manufacturing License
This license is issued to companies involved in producing medicines in India. It ensures that the manufacturer adheres to all necessary standards related to safety, cleanliness, and product quality throughout the production process.
2. Import License
Companies importing drugs, cosmetics, or medical devices into India must obtain this license. It certifies that all incoming products comply with India’s required safety regulations and quality standards.
3. Registration Certificate for Drugs
After a drug is evaluated and approved by CDSCO, a registration certificate is issued. This document authorizes the product to be legally marketed and distributed within India.
4. Medical Device Registration
Before medical devices can be offered in the Indian market, they must be registered with CDSCO. This registration process verifies that the device is safe, reliable, and functions as intended.
5. Clinical Trial Approval
Companies planning to test new medicines or medical products on human participants must obtain prior approval from CDSCO. This approval ensures that the trials follow ethical standards and established scientific protocols.
6. Good Manufacturing Practice (GMP) Certification
GMP certification is granted to pharmaceutical manufacturing units that maintain strict standards of hygiene, safety, and quality throughout the production process. It ensures that the medicines produced are consistent, safe, and effective for consumers.
7. Certificate of Pharmaceutical Product (COPP)
This certificate is required when a company intends to export medicines from India. It confirms that the product is authorized for sale in the country and complies with all applicable regulatory and quality standards.
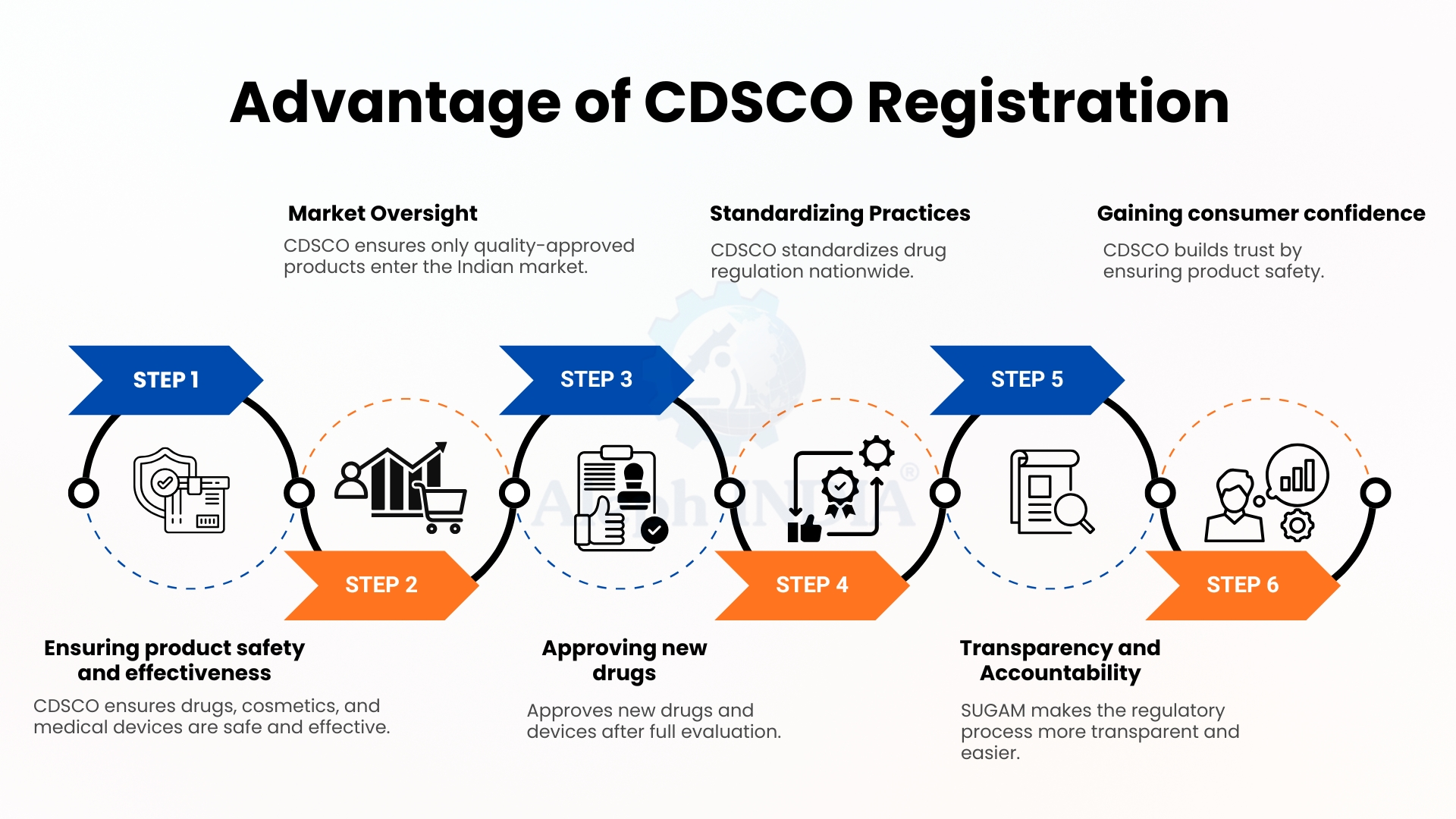
Advantage of CDSCO Registration
The advantages of CDSCO registration include regulation of a wide range of products such as medical devices (non-notified and notified across Class A, B, C & D), cosmetic products, import and manufacturing licenses, as well as test licenses and free sale certificates.
CDSCO registration benefits the Indian market by protecting it from the proliferation of poor-quality and unsafe drugs, devices, and other products. CDSCO registration is valuable because it provides better quality and safer products, as each product is closely monitored and tested to ensure its effectiveness. CDSCO plays a vital role as a border security gateway in the Indian market. Registration of new and existing products is valuable because all new products are thoroughly reviewed. This review of the country's poor quality ensures uniformity in all regulations and guidelines. Through its SUGAM online portal, the organization promotes transparency and streamlines the regulatory process for businesses. CDSCO has enhanced and improved the security of India's healthcare system.
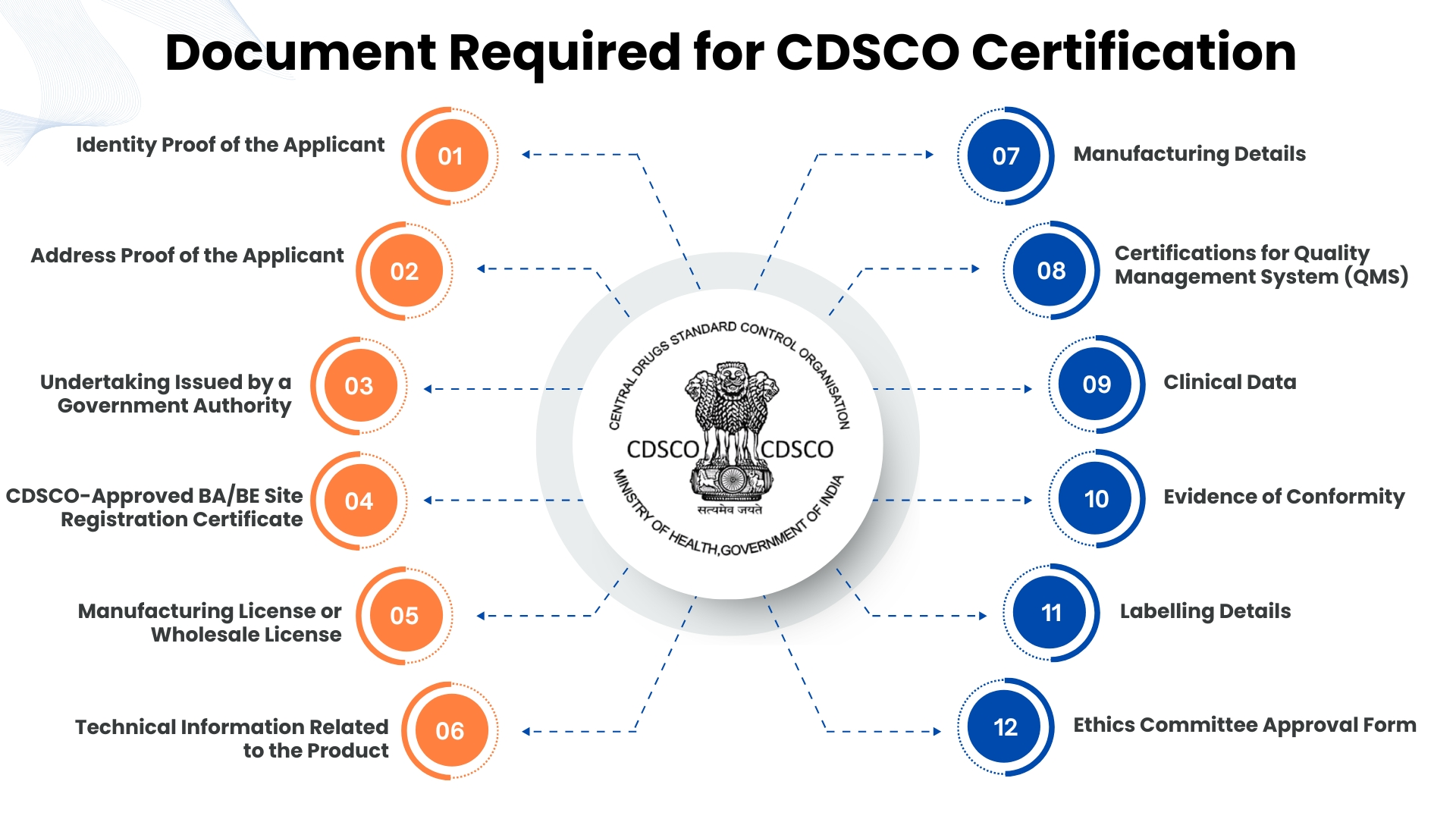
Document Required for CDSCO Certification
To obtain CDSCO certification, it is important to present the specifics of the products alongside the classification, along with a technical document like DMF/PMF for medical devices, Free Sale Certificate, or Market Authorization. Other certifications required for medical devices include ISO 13485; for cosmetics, it’s the GMP certificate; in addition, the device’s label artwork or the list of cosmetic ingredients is required; the undertaking and the authorized representative details for importers; and QMS documents according to CDSCO requirements.
1. Identity Proof of the Applicant
To verify the applicant's identity, a government-issued photo ID such as Aadhaar card, passport, PAN card, or voter ID must be provided.
2. Address Proof of the Applicant
Address proof of the applicant, such as an Aadhaar Card, utility bill, or bank statement displaying the permanent address, must be submitted.
3. Undertaking Issued by a Government Authority
A declaration or undertaking, issued or attested by a competent government authority as per CDSCO requirements, confirming that the applicant agrees to follow all applicable regulatory standards.
4. CDSCO-Approved BA/BE Site Registration Certificate
Manufacturers handling certain pharmaceutical products must obtain BA (Bioavailability) or BE (Bioequivalence) Site Registration, and a valid certificate of the same must be submitted.
5. Manufacturing License or Wholesale License
Applicants are required to submit a valid license issued by the State Licensing Authority for the manufacturing or wholesale distribution of drugs or medical devices.
6. Technical Information Related to the Product
This covers the product’s specifications, design, composition, intended use, and all essential technical details required for registration.
7. Manufacturing Details
Details about the manufacturing site, process flow, quality control measures, and the raw materials used in production.
8. Certifications for Quality Management System (QMS)
ISO 13485 or equivalent certifications demonstrating compliance with national or international quality management standards for medical device or pharmaceutical manufacturing.
9. Clinical Data
For products that require safety and efficacy validation, clinical trial data (Phase I, II, and III) must be submitted, particularly for new drugs, vaccines, or high-risk medical devices.
10. Evidence of Conformity
This includes test reports, performance evaluation reports, or compliance certificates demonstrating that the product meets relevant Indian or international standards such as BIS or ISO.
11. Labelling Details
Sample labels displaying the product name, contents, manufacturer details, batch number, expiry date, usage instructions, warnings, and all other regulatory markings required by CDSCO.
12. Ethics Committee Approval Form
For clinical trials or studies involving human subjects, approval from a registered Ethics Committee must be submitted.
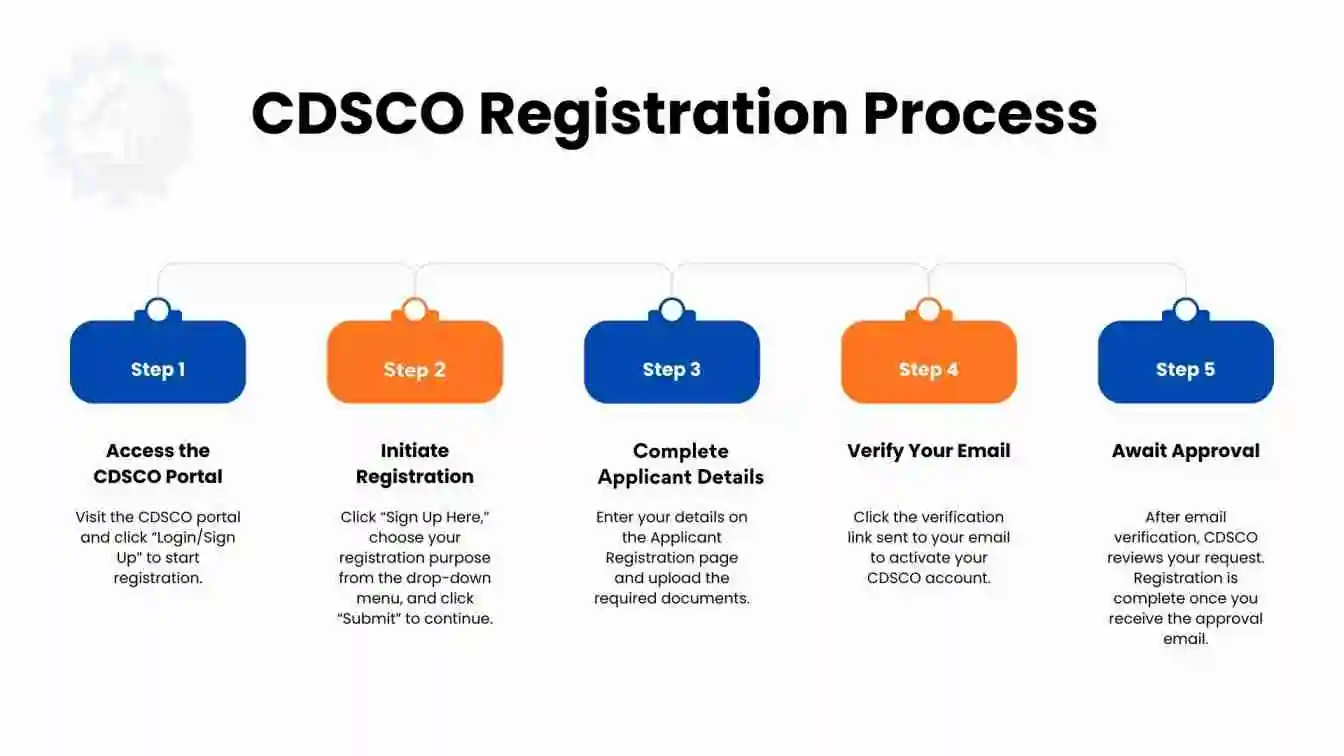
CDSCO Registration Process
Here are the steps for obtaining online registration under the CDSCO portal, which include determining the correct product classification, reviewing documentation through a gap assessment, preparing the DMF or PMF, submitting the application on the SUGAM portal, addressing any CDSCO queries, receiving the registration or license, and completing all post-compliance and renewal activities.
Step 1: Access the CDSCO Portal
Visit the official CDSCO registration portal and select the “Login/Sign Up” option available at the top left corner of the homepage to begin the registration process.
Step 2: Initiate Registration
Click on “Sign Up Here” to begin the registration process. You will be directed to the Registration Purpose page, where you must select the appropriate purpose from the drop-down menu. Once selected, click “Submit” to move to the next step.
Step 3: Complete Applicant Details
You will now be directed to the Applicant Registration page. Begin by entering the following information:
- Applicant Type
- User Name
- Password
- Name
- Mobile Number
- Email ID
Step 4: Verify Your Email
A verification link will be sent to your registered email address. Open the email and click on the link to confirm your details and activate your account on the CDSCO portal.
Step 5: Await Approval After verifying your email, your registration request is forwarded to the concerned CDSCO officials for review. You will be notified via email once your application is either approved or requires further action. Your registration is considered complete only after you receive the official approval email.
Product-wise Fees For CDSCO License Registration
Registration costs by CDSCO It depends on type of product category and approval you are looking for. The fees structures for each category like drugs, medical devices and cosmetics are defined as per the CDSCO guidelines. Import registrations usually attract higher fees as they are subjected to a greater level of scrutiny, and the manufacturing licenses have varying slab based fees on device class or product type. Costs can also raise if there are more products, versions or testing requirements. It's very important to know what the right category and fee are for proper budgeting as well as an easy approval. Manufacturers and importers are encouraged to review CDSCO’s current fee structure when beginning the application process.
Medical Devices
CDSCO registration fees for medical devices vary according to device class and whether the application is for manufacturing or import, and these fees are approximate values.
| Manufacturing License Fees (Class A & B Devices) |
| License Fee |
5,000 INR |
|
| Device Fee |
500 INR |
Per Device |
| Manufacturing License Fees (Class C & D Devices) |
| License Fee |
50,000 INR |
|
| Device Fee |
1,000 INR |
Per Device |
| Import License Fees |
| Class A (Sterile/Measuring) |
83,300 INR (Per Site) |
4,165 INR (Per Product) |
| Class B Devices |
1,66,600 INR (Per Site) |
83,300 INR (Per Product) |
| Class C & D Devices |
2,49,900 INR (Per Site) |
1,24,950 INR (Per Product) |
Drugs
| First Product |
10,000 INR |
|
| Each Additional Product |
1,000 INR |
|
| Registration Certificate (Form 40) |
| Manufacturing Premises |
8,33,000 INR |
|
| Single Drug (Import & Indian Use) |
4,16,500 INR |
|
| Each Additional Drug |
4,16,500 INR |
|
Cosmetics
| Up to 10 Items Per Category |
10,000 INR |
|
| Each Additional Item |
500 INR |
|
| Cosmetics – Import Registration Certificate (Form COS–4A) |
| First Category |
83,300 INR |
|
| Each Additional Category |
83,300 INR |
|
| Each Variant |
4,165 INR |
|
| Manufacturing Site Registration for Imports |
| Per Manufacturing Site |
41,650 INR |
|
Time Period for CDSCO Certification
The duration for obtaining CDSCO Certification in India will depend on product type, risk classification and completeness of documents. Overall, the approval time frame varies from 1 to 9 months although complex or innovative products may require more time.
Low risk Class A and B medical devices are generally cleared within 1-3 months, for the reason that the regulatory pathway for this class of products is relatively easy and can be self-certified on CDSCO portal. On the other hand, higher risk Class C and D medical devices are subjected to comprehensive technical and clinical assessment; thus, their licensing process could require 3-6 months, with most needing up to 6-9 months.
Registration of the cosmetic product is normally 6-9months Import licenses is then about another 4 to 6 month inclusive of submitted identifiers and cases.
It is also important to note that the processing time will be directly affected by how complete and accurate your application is. Additionally there is a time where review stops if the Agency has questions of a regulatory nature till such questions are answered to their satisfaction and documented, potentially adding weeks or months to your timeline. Applications for products with novel technology, high risk devices or without predicate device will also be referred to the Subject Expert Committee (SEC) / Medical Device Advisory Committee (MDAC). In these situations, the overall time to approval may be 9–12 months or even longer.

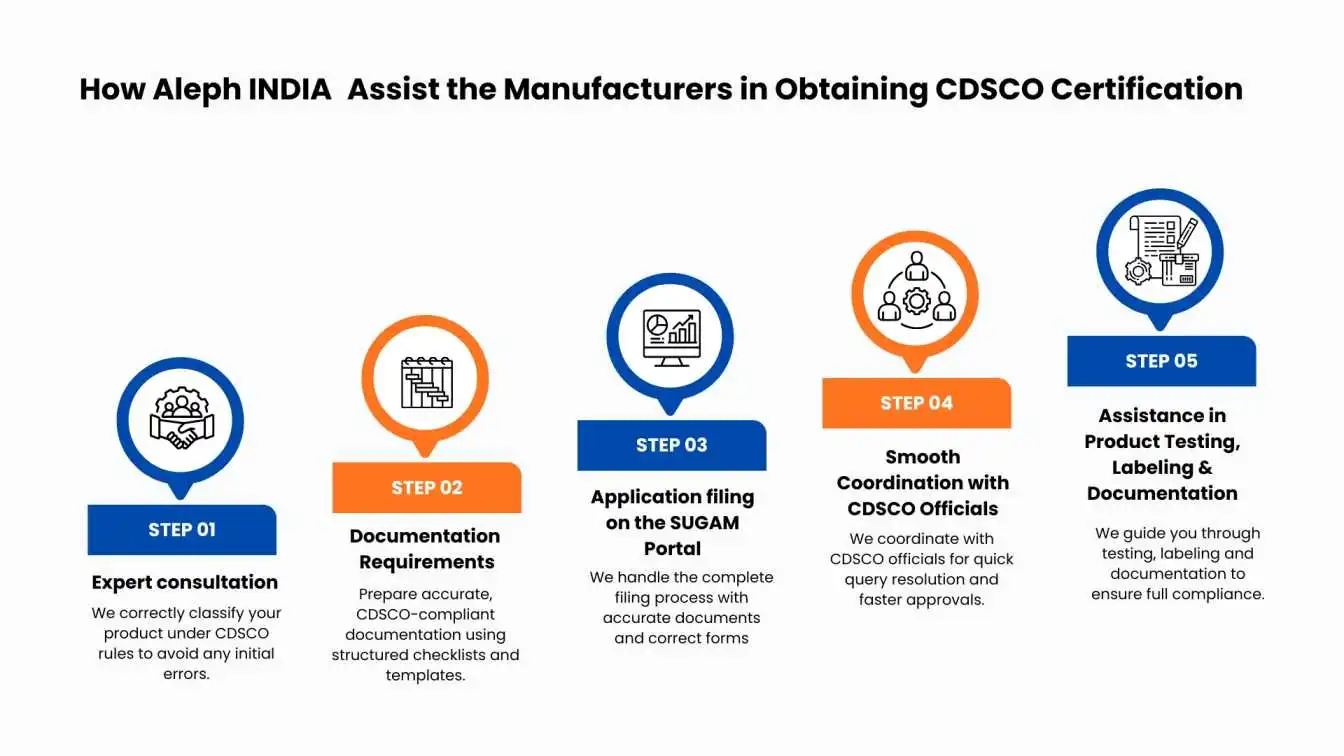
How Aleph INDIA Assist the Manufacturers in Obtaining CDSCO Certification
Obtaining CDSCO certification in India is quite a complex process consisting of thick documentation and various procedural steps. Approvals are a problem because guidelines are unclear, there are delays and gaps between what is legal. Aleph INDIA fills the gaps with end-to-end regulatory assistance ensuring a hassle-free, cost-effective and compliant certification process.
1. Professional Regulatory Consulting & Product Classification
Challenge : Device class or the cosmetic category to which it belongs is probably the most critical step. Misclassifying means a rejection of the application or significant delay.
Solution By Aleph INDIA : We analyze the product, map it to CDSCO rules and classify the product correctly which makes no chance for the errors even at initial stage.
2. Documentation Requirements
Challenge: CDSCO registration involves a lot of documentation, such as Device Master Files (DMFs), Plant Master Files (PMFs), and other technical and administrative documents.
Solution: Prepare detailed and accurate documentation that meets the requirements of the CDSCO license. Use checklists and templates to ensure proper document formatting.
3. Complete Application Filing on the SUGAM Portal
Challenge : Most of the applications are encountered with queries or rejection on account of either wrong filling-up form or attachments not being properly attached on the SUGAM portal.
Role of Aleph INDIA:We manage the entire filing process for you, from submitting proper documentation to selecting the appropriate forms to ensuring application completeness.
4. Seamless Coordination With CDSCO Officials
Challenge :Delays often occur due to queries, document clarifications, or communication gaps with authorities.
What Aleph INDIA Does:Our team proactively coordinates with CDSCO officials, addresses queries promptly, and ensures faster processing and approval.
5. Assistance in Product Testing, Labeling & Documentation
Challenge :Goods are rejected due to mislabeling, oversight of caution notice or test report.
Aleph INDIA Support:We guide you through testing, labeling and documentation to ensure full compliance
6. Post-Certification Compliance, Renewals & Audits
Challenge :Continued compliance is required following approval. Renewals and audits are a pain point for many companies.
How Aleph INDIA Assists: We offer on-going support for post-market compliance, timely renewals, audit preparedness, amendments and notifications as regulations evolve.
7. Complete Support for BIS, Legal Metrology & Other Regulatory Needs
Challenge:It is common for manufacturers to require multiple certifications in addition to CDSCO, which causes confusion and results in disparate management.
Aleph INDIA Advantages: We provide one-stop solution for BIS, LMPC, WPC, EPR and FSSAI & all-Round India Compliance with our multi- regulatory capabilities.